

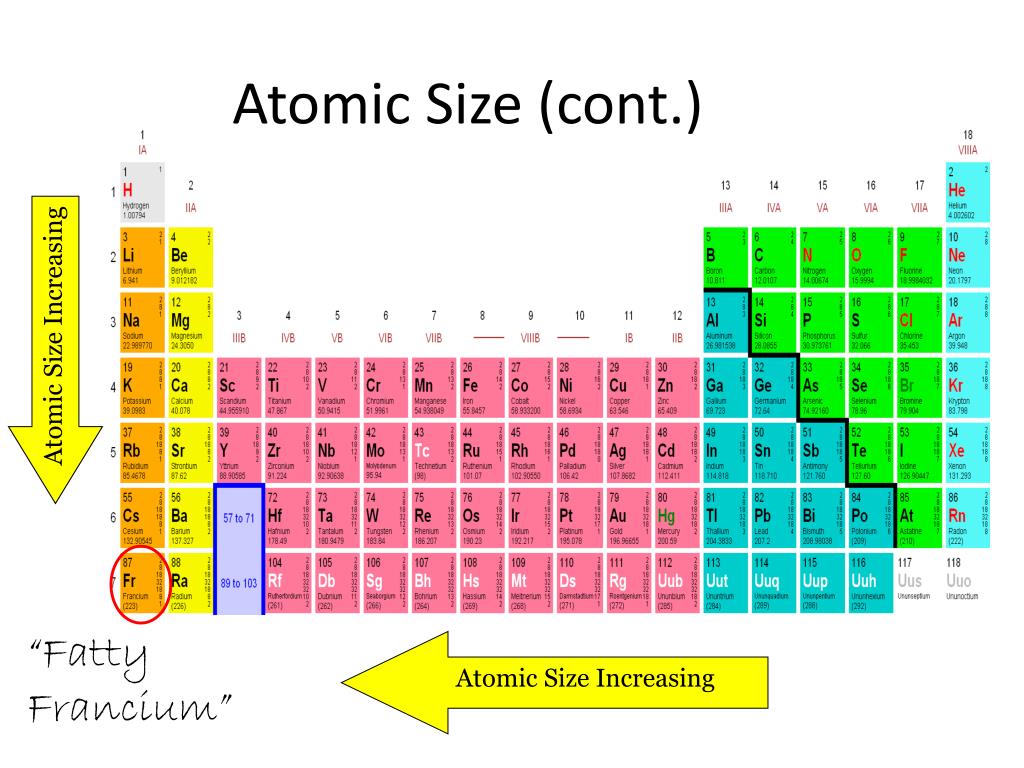
Model 1: Atomic radii in groups I and II of the periodic table: 1 Slater, J.C., J. 5.0 (1 review) Get a hint What is the periodic trend for atomic size from top to bottom in a group from left to right in a period Click the card to flip The atomic size increases from top to bottom and decreases from left to right. Compare your predictions to the values given in model 1 below. Consistent with size trends, first ionization energies generally increase across a period and decrease down a group. Predict the trend for the atomic radius for the elements in group I and group II of the periodic table. Atomic size measured the distance between the nucleus of an atom and the outermost non-valence electrons of the atom. Trends are based on Coulombs law which mathematically relates several characteristics of an elements. Explain why you see this trend as you move across a period. Does the same trend hold true Is this the trend you predicted Yes. Choose a couple more periods (excluding 1, 6, and 7). There are some outliers (not addressed in this worksheet). The valence electrons are closer to the nucleus to which they are attracted in a smaller atom thus, more energy will be required to remove an electron by ionization. Periodic trends predict differences between elemental characteristics as you move across the periodic table. It trends downward so the atomic radius gets smaller across a period. Trends in first ionization energies can be understood on the basis of size of atoms.
\Īll ionization energies are positive, because it takes energy to remove an electron from the attraction of an atom’s nucleus. The first ionization energy, \(I_1\), refers to removing one electron from a neutral atom:

Ionization energy, I, is the energy required to remove an electron from a gaseous species. In the chart below, note the trends in the sizes (radii in picometers, pm) of the following ions with the same charge. Alkanes are simplest organic compounds that consist of single bonded carbon and hydrogen atoms with the general formula CnH2n+2. What trend in the atomic radius occurs down a group on the periodic table What causes this trend Ba. Ionic radii increase down a group for ions of the same charge.


 0 kommentar(er)
0 kommentar(er)
